The MHRA has issued a further warning about the risks of taking valproate during pregnancy in April 2017
Babies born to mothers who take valproate medicines (Epilim▼, Depakote▼) during pregnancy have a 30–40% risk of developmental disability and a 10% risk of birth defects. Despite communications to prescribers in January 2015 and February 2016 on the magnitude of this risk and the actions to take, there is evidence that women are still not aware of the risk.
More information can be found on the MHRA website.
MHRA advice to healthcare professionals
- Do not prescribe valproate medicines for epilepsy or bipolar disorder in women and girls unless other treatments are ineffective or not tolerated; migraine is not a licensed indication.
- Ensure women and girls taking valproate medicines understand the 30–40% risk of neurodevelopmental disorders and 10% risk of birth defects and are using effective contraception.
- Valproate use in women and girls of childbearing potential must be initiated and supervised by specialists in the treatment of epilepsy or bipolar disorder
QMasters' tools to help GP practices implement this guidance
Please download our free search to identify pregnant patients who are taking valproate.
You must right-click on this and select "Save As" and import the file into Population Reporting in EMIS Web.
You must right-click on this and select "Save As" and import the file into Population Reporting in EMIS Web.
| valproate__v7.3_.xml |
Please note our search relies on accurate clinical coding to identify these patients and we are unable to guarantee that all patients will be correctly identified.
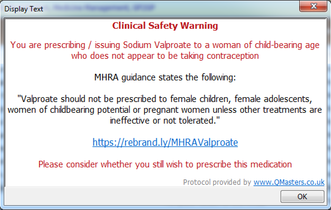
GP practices subscribing to our QToolset will receive an automatic clinical safety pop-up warning with their next update. This warning will appear whenever valproate is issued for a woman of childbearing age who is not receiving contraception, or who are pregnant.
For more information about our QToolset and to learn how it will save GPs time, maximise income, and improve clinical safety please visit www.QMasters.co.uk/qtoolset.

 RSS Feed
RSS Feed
