The MHRA have strengthened and updated their guidance for Valproate in women of childbearing age. There is now an absolute contraindication unless a pregnancy prevention programme is in place. For more details fo what this means, please see:
https://www.gov.uk/drug-safety-update/valproate-medicines-epilim-depakote-contraindicated-in-women-and-girls-of-childbearing-potential-unless-conditions-of-pregnancy-prevention-programme-are-met
QMasters' tools to help GP practices implement this guidance
QMasters' safety search for valproate will help identify patients at risk.
Please download our free search to identify pregnant patients who are taking valproate.
You must right-click on this and select "Save As" and import the file into Population Reporting in EMIS Web.
https://www.gov.uk/drug-safety-update/valproate-medicines-epilim-depakote-contraindicated-in-women-and-girls-of-childbearing-potential-unless-conditions-of-pregnancy-prevention-programme-are-met
QMasters' tools to help GP practices implement this guidance
QMasters' safety search for valproate will help identify patients at risk.
Please download our free search to identify pregnant patients who are taking valproate.
You must right-click on this and select "Save As" and import the file into Population Reporting in EMIS Web.
| valproate_pregnancy__v8.0_.xml |
Please note our search relies on accurate clinical coding to identify these patients and we are unable to guarantee that all patients will be correctly identified.
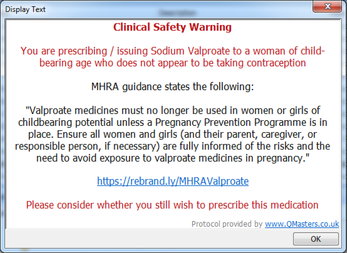
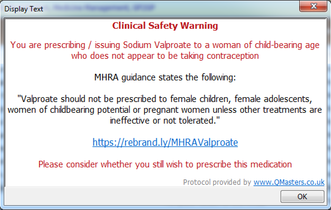
GP practices subscribing to our QToolset will receive an updated clinical safety pop-up warning with their next update. This warning will appear whenever valproate is issued for a woman of childbearing age who is not receiving contraception.
GP practices subscribing to our QToolset will receive an updated clinical safety pop-up warning with their next update. This warning will appear whenever valproate is issued for a woman of childbearing age who is not receiving contraception.

 RSS Feed
RSS Feed
